Abstract
Background: Chronic myeloid leukemia (CML) is the commonest leukemia in adult population in India. The advent of tyrosine kinase inhibitors (TKI) targeting BCR-ABL1 fusion transcript in CML has dramatically improved survival of CML patients. Generic formulations of imatinib mesylate have been the mainstay of TKI therapy in Indian CML patients for more than a decade, due to prohibitive costs of 2nd generation TKIs and poor socio-economic status of majority of patients. However, with the availability of generic dasatinib in India since March 2021, the cost of dasatinib therapy has become comparable to that of generic imatinib treatment, thereby enabling more extensive use of dasatinib as first-line TKI therapy in CML patients in India. Achievement of early molecular response (EMR) after 3 months of first-line TKI therapy, defined as blood BCR-ABL1 transcript level ≤ 10% [international scale (IS)], has emerged as an important predictor of favorable long-term outcome in CML-CP. There is scarcity of data on efficacy & safety of generic formulations of dasatinib vis-à-vis generic imatinib in CML-CP patients in India, which the present study has attempted to explore at a tertiary care hospital in India.
Objectives: The objectives of the study were to compare early molecular response (EMR) [defined as BCR-ABL1 transcript level ≤ 10%, I.S.] at 3 months of therapy with generic dasatinib versus generic imatinib in newly diagnosed CML-CP patients and to compare treatment-related adverse effects in the two groups.
Methods: This randomized controlled study enrolled newly diagnosed CML-CP patients after obtaining institutional ethics committee approval & informed consent.
Inclusion Criteria:
Newly diagnosed patients with chronic phase CML.
Age ≥ 12 years.
Exclusion Criteria:
Patients with de novo accelerated phase or blast phase CML.
Pregnancy & lactation.
Patients with pre-existing pleural effusion, grade ≥ 3 lung disease, or pulmonary arterial hypertension.
Patients with angina, or grade ≥ 3 cardiac disease [as defined by the New York Heart Association (NYHA) criteria] at diagnosis.
Patients with prolonged QTc interval (> 480 msec) at baseline.
The EUTOS long-term survival (ELTS) risk score was calculated at diagnosis. Patients were randomized to one of the two following treatment groups:
Group A: Dasatinib 100 mg p.o. once daily (60 mg/m2/day for age <18 years).
Group B: Imatinib 400 mg p.o. once daily (340 mg/m2/day for age < 18 years).
Follow up & monitoring of patients was done every 2 weekly till completion of 3 months of TKI therapy, followed by assessment of hematological response & molecular response as per standard criteria.
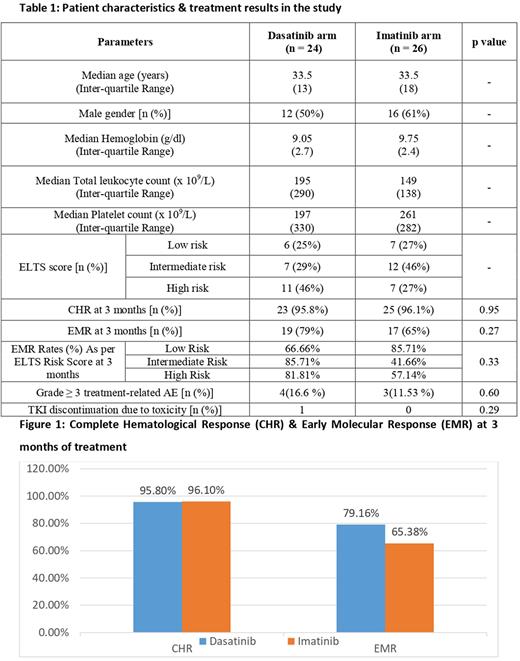
Results: A total of 61 consecutive CML patients were screened between June 2021 and March 2022, out of which 54 patients with confirmed diagnosis of CML-CP were enrolled in the study and were randomized to receive generic dasatinib or generic imatinib as first-line therapy and were followed up for 3 months for EMR and complete hematological response (CHR). Four patients were lost to follow up and data was analyzed for 50 patients for whom EMR results at 3 months was available. Out of these 50 patients, 24 patients received dasatinib & 26 patients received imatinib. The median age of patients was 33 years (range 18-64 years), with male: female ratio of 1.3: 1. Achievement of CHR was documented in 96% of patients in both dasatinib & imatinib treatment arms. EMR at 3 months was achieved in overall 72% patients in study [19/24 patients (79%) in dasatinib arm & 17/26 patients (65%) in imatinib arm]. However, the difference was not statistically significant (p = 0.27). Notably, EMR rates in ELTS intermediate-risk group was 86% in dasatinib arm versus 42% in imatinib arm, and EMR rates in ELTS high-risk group was 82% in dasatinib arm versus 57% in imatinib arm (p = 0.33). The incidence of grade 3-4 adverse events was not significantly different in the two treatment groups. The patient characteristics & treatment results are summarized in Table 1 & Figure 1.
Conclusion: The results of the present study suggested that generic formulations of dasatinib were well-tolerated and were associated with improved early molecular response rates compared to generic imatinib in CML-CP patients in India.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal